Ultrasound-Assisted Lumbar Puncture in Infants
Panida Kanjanauptom, MD, Pediatric Emergency Ultrasound Faculty, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Almaz Dessie, MD, Assistant Professor of Emergency Medicine and Pediatrics, Alpert Medical School of Brown University, Providence, RI
Introduction
Lumbar puncture (LP) is a common procedure in febrile or septic infants presented to the Emergency Department. Performing an LP can be challenging in infants with failure rates as high as 65% among novice clinicians and 37% in experienced hands.1,2
Ultrasound (US)-assisted LP in infants has a fair amount of literature regarding its utility, with some studies showing it offers significant advantage in reducing the risk of traumatic tap compared to traditional landmark palpation technique.3 Although a small meta-analysis did not show an overall reduced risk of LP failure, the rate of first attempt success of US-assisted LP in some studies was higher than that of LP using standard technique.3-5 Furthermore, performing US-assisted LP is technically feasible and quickly performed even by novice sonographers and is well accepted by clinicians and family.6 US-assisted LP can also affect clinician decision-making on the selected level of LP by clearly delineating anatomy and identifying a safe needle insertion site, which may be higher or lower than sites identified by traditional palpation technique alone.6
Anatomy
Minimal body fat and incompletely ossified spinous processes allow for easy visualization of the spinal cord anatomy in infants using ultrasound. The spinal cord tapers into a caudal end at the conus medullaris (CM), from which originate the fibers around it, the cauda equina (CE). It is important to locate the end of CM before performing LP as, above this point, the LP needle can cause damage to the spinal cord. Clinicians often use landmark technique, in which the superior border of the iliac crests is palpated to mark an intercristal line (ICL) and the intervertebral space above it is used for needle insertion. By using the ICL as a landmark, the intended intervertebral level for LP is between L3/4 or L4/5 intervertebral space, well below the expected termination point of the CM between the L1/2 intervertebral space and the inferior boarder of L2 vertebra. However, one study demonstrated the potential site for LP identified by manual palpation using landmark technique varies greatly in term neonates and may be inaccurate. Moreover, the tip of the CM in term neonates ends lower than the expected vertebral level in approximately 40%.7 Also, the terminal location of CM in premature infants may be quite inferior. A study in ex-preterm infants, with a corrected gestational age of 60 weeks or less, revealed the inferior tip of the CM is often located just above the L3/4 intervertebral space.8
There are two main blood vessels that supply the spinal cord. In terms of arteries, there is one anterior spinal artery and two posterolateral spinal arteries, while the venous blood supply is located in both the ventral and dorsal epidural space.
Technique
US-assisted LP is performed using the high-frequency linear transducer at the infant’s lower lumbar spine, with the patient either in a lateral recumbent position or an upright sitting position. The standard LP position, in which the neck and hips are flexed, should be used to locate the optimal site for needle insertion. Warm gel should be used during US to provide comfort and reduce the risk of hypothermia in infants. Align the transducer along the spinous process of the lumbar spine above the level of intercristal line to obtain a sagittal view of the spinal canal. The tapered CM is identified, below which any space is safe for needle insertion. The amount of anechoic cerebrospinal fluid (CSF) can be grossly compared in each space to locate the ideal LP site to obtain maximal CSF volume. (Figure 1A-B) The next step is to mark the skin at this optimal intervertebral level using a marking pen. Additionally, we recommend measuring the depth from the skin to the posterior border of the subarachnoid space using calipers, ensuring an angle of entry of 30–45 degrees. (Figure 1C) This allows the proceduralist to more precisely estimate the minimum needle depth needed to reach the subarachnoid space.
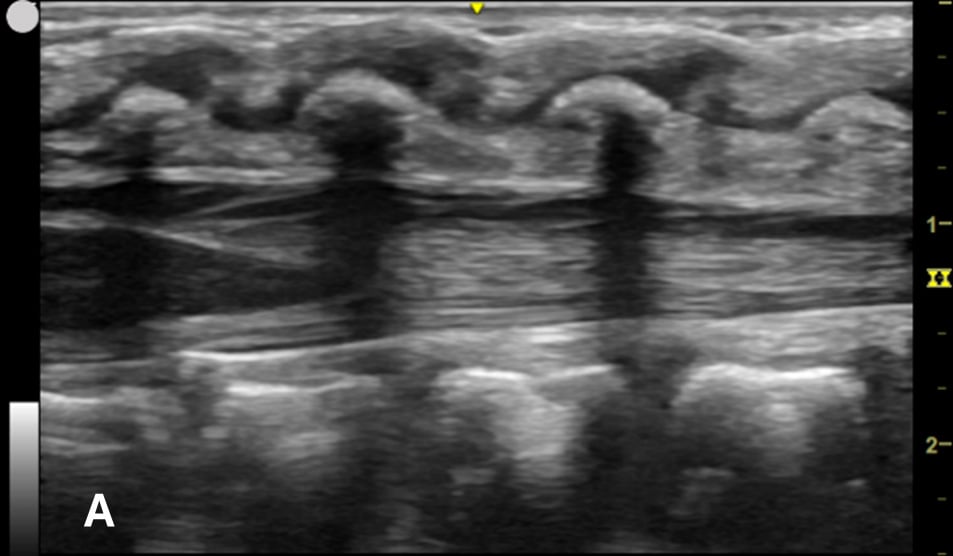
Figure 1A - Corresponding US image showing the target area of the lower spinal canal in sagittal/longitudinal view.
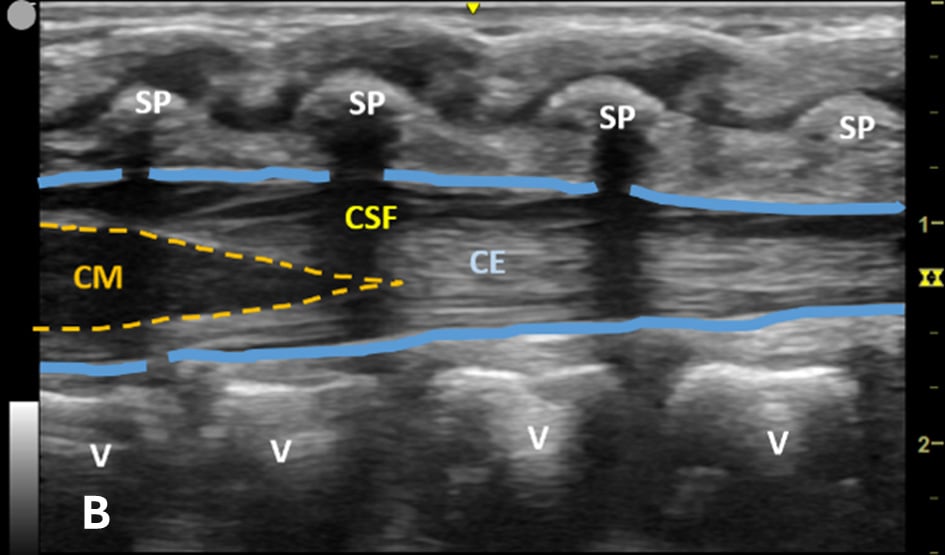
Figure 1B - Structures in the target area: SP (spinous process), CSF (cerebrospinal fluid), CM (conus medullaris), and CE (cauda equina).
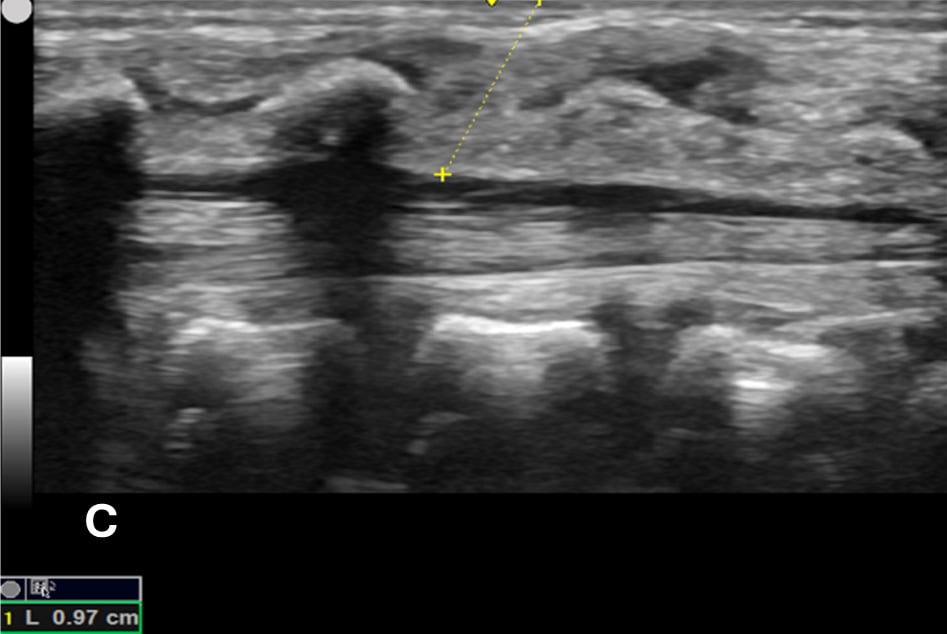
Figure 1C - The minimum depth from the skin to the posterior border of subarachnoid space for needle entry using calipers, which is measured at 0.97 cm (yellow dotted line).
The transverse view should also be obtained to verify landmarks and identify any overlying vessels or hematomas (from prior attempts) to avoid. The transducer is rotated to the transverse position on the lower back and moved caudally over the spinous processes to locate the CM, which is a hypoechoic structure positioned in the center of the spinal canal. At higher levels of the CM, the ventral roots and dorsal roots can be identified, which are surrounded by the anechoic CSF. Next, gradually move the transducer caudally and observe the CM decreasing in size while being encircled by the hyperechoic fibers of the CE. (Figure 2) Once this sonographic landmark is identified, locate the center of the transducer and mark the corresponding area on the skin, aligning it with the center of the spinal cord. Subsequently, draw lines from both markers in both planes and utilize the point of intersection as a guide for inserting the LP needle. To enhance visualization of the spinal cord’s vascular supply, and avoid a space with any overlying vessels that may cause a traumatic tap, it is advisable to apply color Doppler in both the sagittal/longitudinal and transverse views.9 (Figure 3)
Figure 2 - Corresponding US image showing spinal canal structures in transverse view:
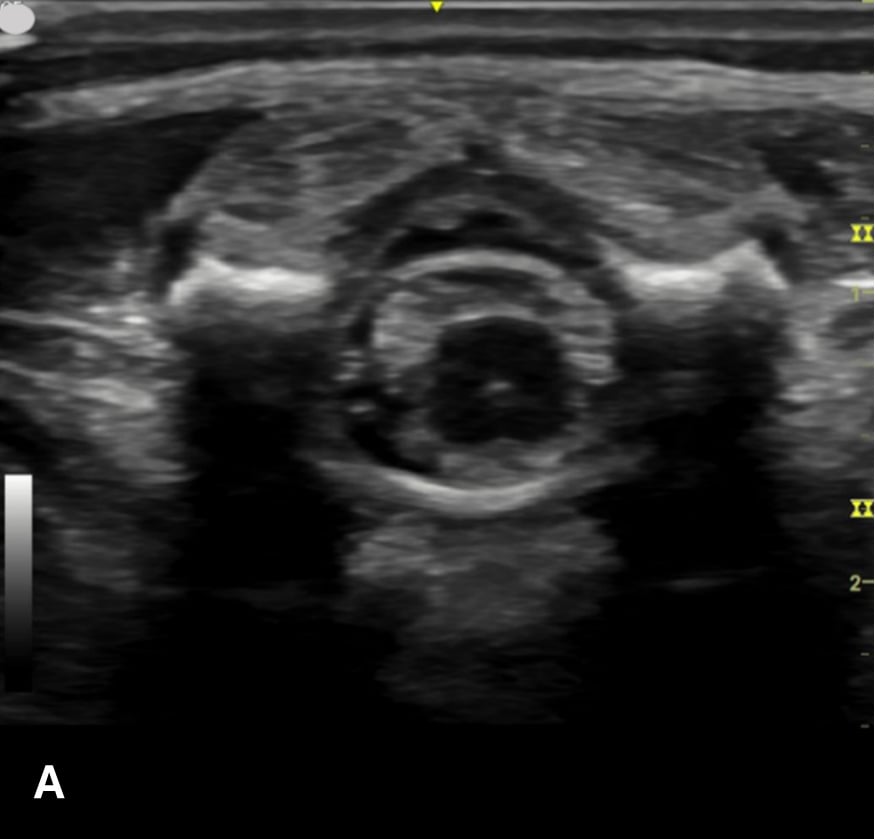
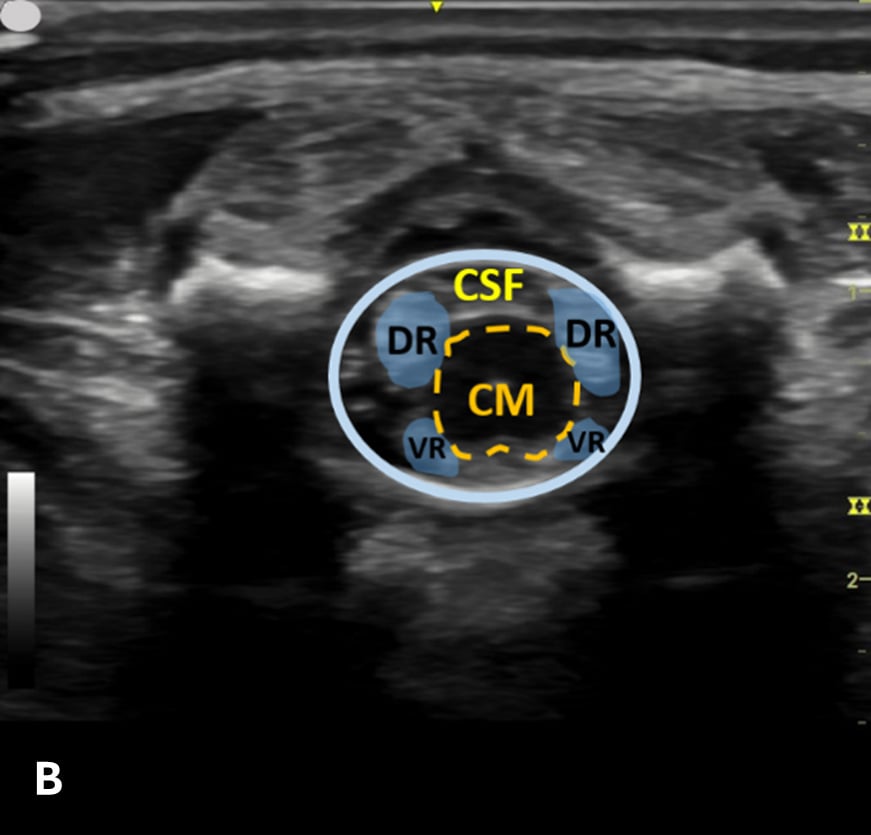
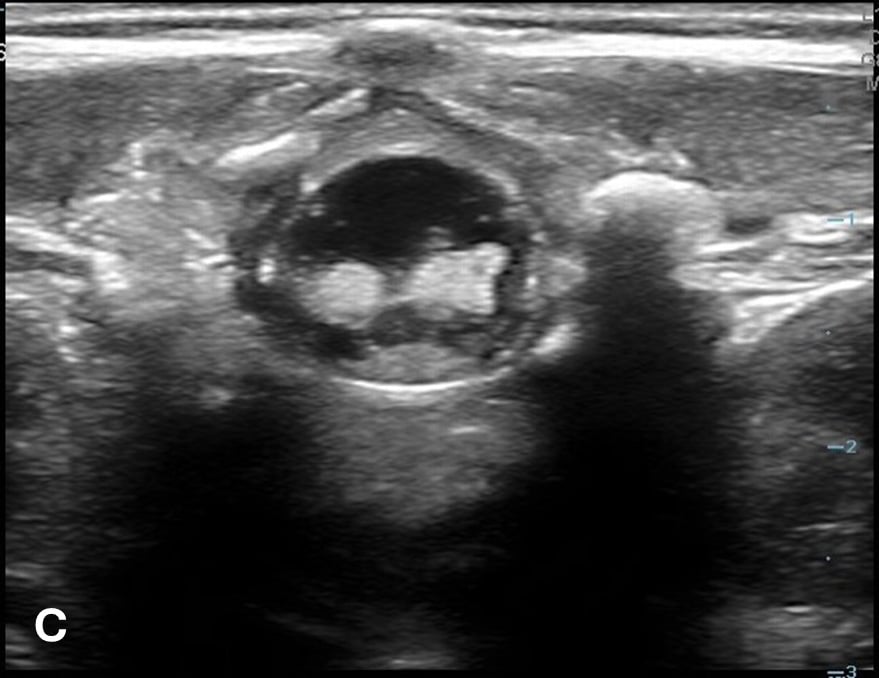
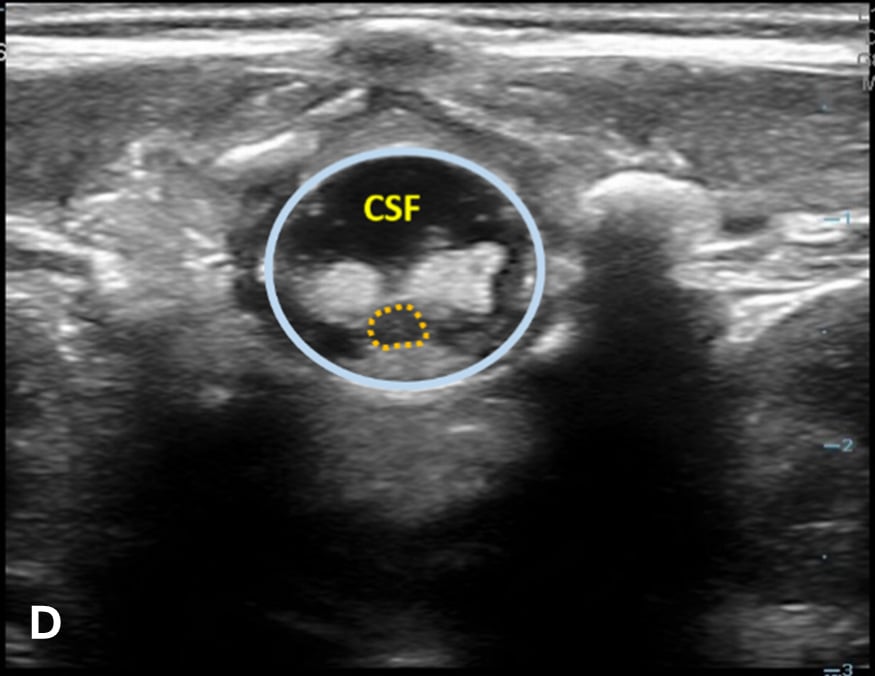
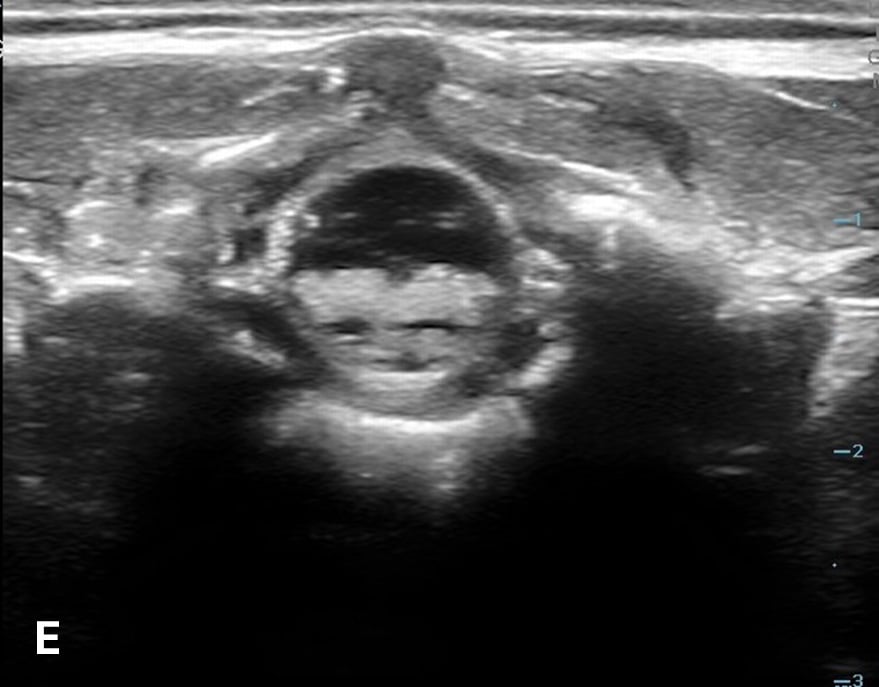
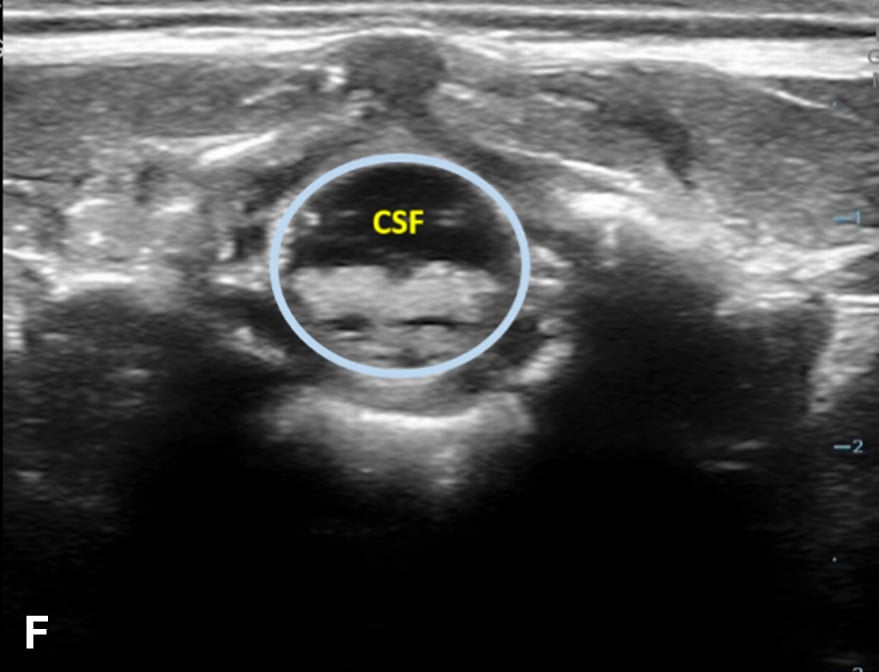
Figure 2 - Corresponding US image showing spinal canal structures in transverse view. 2A-B: The caudal portion of the transverse view shows DR (dorsal roots), VR (ventral roots), CSF (cerebrospinal fluid), and CM (conus medullaris). 2C-D: The probe is moved caudally demonstrating the tapered CM (orange dotted circle), CE (cauda equina, hyperechoic structure surrounding the CM) and a pocket of CSF. 2E-F: Demonstrate the disappearance of the CM, and the pocket of CSF becoming larger as the probe was moved caudally. The blue circle is the entire dural sac encircling the spinal canal.
Figure 3 - Color Doppler US image of the spinal canal:
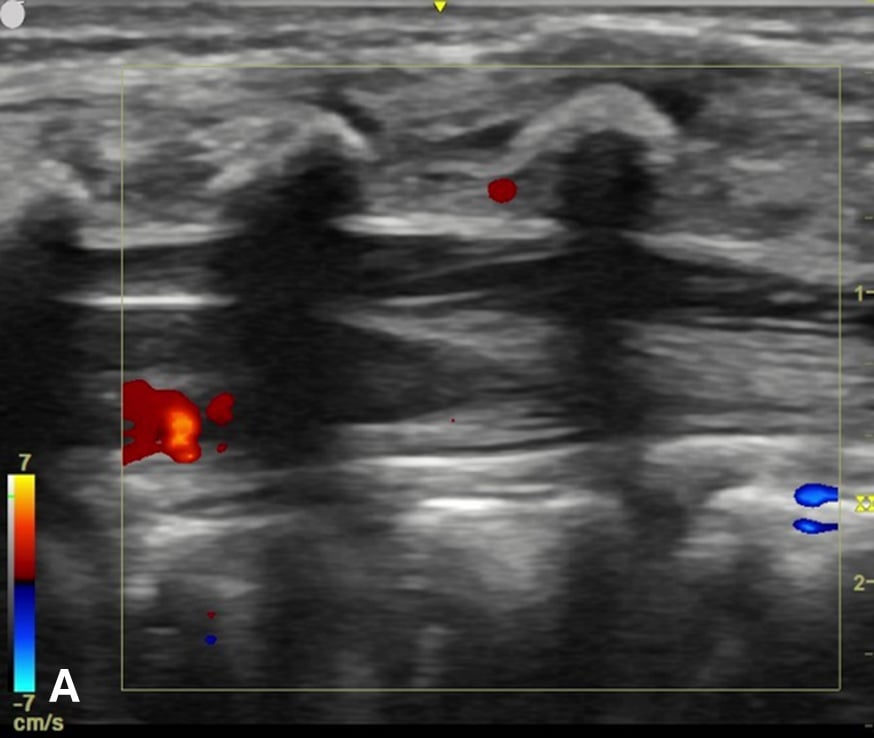
Figure 3A - The sagittal view demonstrates no prominent spinal vessels.
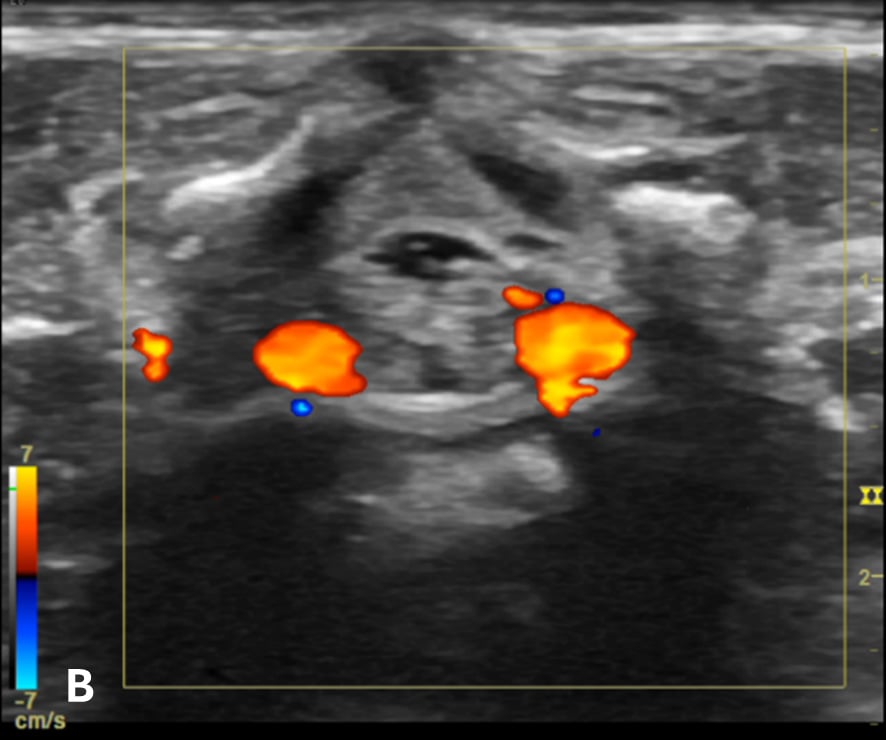
Figure 3B - The transverse view shows the anterior spinal blood vessels at the lateroventral part of the spinal canal.
Discussion
US-assisted LP is useful in assessing the spinal canal structures before performing LP in young infants. Preprocedural US may help clinicians reduce the number of failed attempts, estimate needle depth, avoid a traumatic tap, and select an ideal site for safe needle insertion, while also being able to be performed quickly by novice sonographers.
US-assisted LP has a growing body of supportive evidence in the evaluation of febrile infants and should be incorporated into routine practice in the ED among ultrasound-trained physicians. We suggest that the evaluation should be conducted before performing the procedure in both routine and previously unsuccessful LP attempts. This approach aims to utilize the emergency physician’s US skills to improve success rates and minimize complications associated with infant LP.
References
- Nigrovic LE, Kuppermann N, Neuman MI. Risk factors for traumatic or unsuccessful lumbar punctures in children. Ann Emerg Med. 2007;49(6):762-71.
- Kessler DO, Arteaga G, Ching K, Haubner L, Kamdar G, Krantz A, et al. Interns' success with clinical procedures in infants after simulation training. Pediatrics. 2013;131(3):e811-20.
- Olowoyeye A, Fadahunsi O, Okudo J, Opaneye O, Okwundu C. Ultrasound imaging versus palpation method for diagnostic lumbar puncture in neonates and infants: a systematic review and meta-analysis. BMJ paediatrics open. 2019;3(1):e000412.
- Neal JT, Kaplan SL, Woodford AL, Desai K, Zorc JJ, Chen AE. The Effect of Bedside Ultrasonographic Skin Marking on Infant Lumbar Puncture Success: A Randomized Controlled Trial. Ann Emerg Med. 2017;69(5):610-9.e1.
- Gorn M, Kunkov S, Crain EF. Prospective Investigation of a Novel Ultrasound-assisted Lumbar Puncture Technique on Infants in the Pediatric Emergency Department. Acad Emerg Med. 2017;24(1):6-12.
- Kessler D, Pahalyants V, Kriger J, Behr G, Dayan P. Preprocedural Ultrasound for Infant Lumbar Puncture: A Randomized Clinical Trial. Acad Emerg Med. 2018;25(9):1027-34.
- Baxter B, Evans J, Morris R, Ghafoor U, Nana M, Weldon T, et al. Neonatal lumbar puncture: are clinical landmarks accurate? Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F448-50.
- Cristiani F, Henderson R, Lauber C, Boretsky K. Success of bedside ultrasound to identify puncture site for spinal anesthesia in neonates and infants. Reg Anesth Pain Med. 2019.
- Halm BM, Kessler DO. Color Flow Doppler Point of Care Ultrasound to Evaluate Vessels before Infant Lumbar Puncture. J Emerg Med. 2017;52(1):70-3.

