Advanced Echo Pocket Card Series: Regional Wall Motion Abnormality
Kanisha Cruz-Kan, MD., FRCPC-EM, University of Manitoba
Muzeen Ismath, MD., FRCPC-EM, University of Manitoba
Chitbhanu Singh, MD., FRCPC-EM, University of Manitoba
Tomislav Jelic, MD, FRCPC, DRCPSC, FACEP, AEMUS-FPD, University of Manitoba
Case Presentation
A 52-year-old healthy female presented to the ED in extremis with shortness of breath. Her initial vital signs were notable for T37.5ºC, HR 110, BP 70/40, RR 40, SpO2 of 89% on 15L. Her prehospital ECG was unremarkable. Shortly upon arrival, the patient suffered rapid deterioration with refractory hypoxemia and altered mentation, necessitating intubation and mechanical ventilation. A bedside TTE was performed to evaluate for etiology of her shock, which showed severely depressed LV function with gross anteroseptal regional wall motion abnormalities (RWMA) noted. Additional findings on TTE included severe MR, bilateral B lines, and a plethoric IVC. No pericardial effusion or evidence of right heart strain was noted.
Background
Identification of RWMA can assist in detection of acute myocardial infarction (AMI) and is included in the 4th universal definition of MI.1 Myocardial ischemia can produce RWMA shortly after coronary artery occlusion, and these changes often precede ECG findings or development of symptoms.2-4 Thus, patients with concerning historical features, equivocal ECG findings, or high pre-test probability may benefit from early focused bedside echocardiography to identify these changes and minimize delays to definitive treatment.
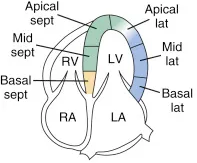
The American Society of Echocardiography (ASE) recommends dividing the LV into 16 anatomic segments; for simplicity and efficiency, this can be further condensed into four focused segments (anterior, septal, inferior, and lateral).5
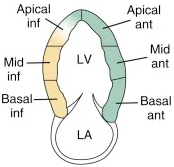
Emergency physicians should develop competency in the interrogation above segments using the parasternal long axis (PLAX), short axis (PSSX), and apical (four-chamber and two-chamber) views. We will be following cardiology convention with respect to screen orientation. PLAX can be obtained at the left 4th-5th parasternal space, with the probe marker towards the R shoulder. Rotating the probe 90º obtains the PSSX. The apical four chamber view is obtained at the point of maximal impulse, with the marker towards the patient’s left side. Rotating the probe 60º generates the apical two chamber view as demonstrated in Figure 1. This view is seldom used in EM practice but does allow for an alternate view of the anterior and inferior left ventricular walls.
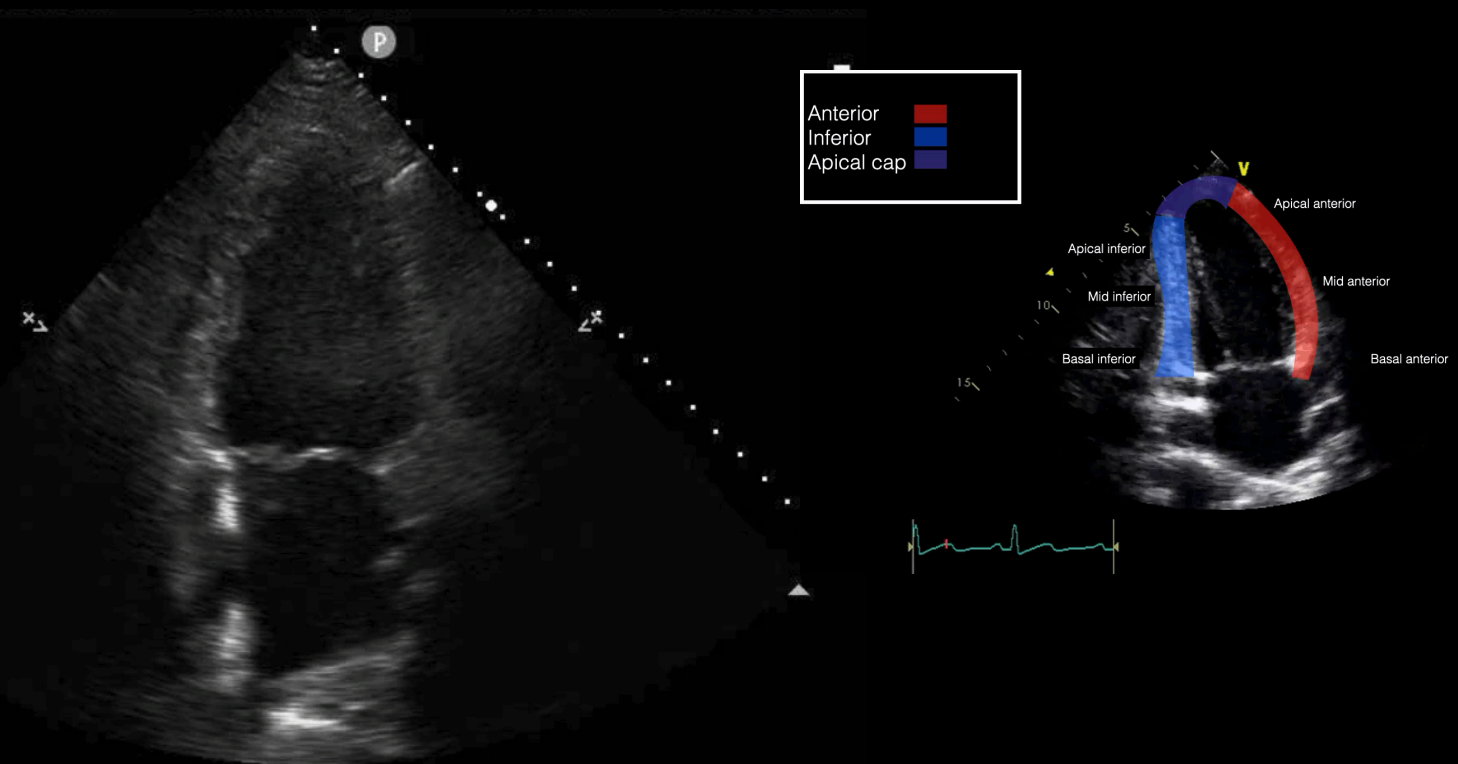
Figure 1 - Apical 2-chamber view and corresponding anatomy
With respect to corroborating the LV regional abnormalities with these views, the PSLX is best suited for anteroseptal and inferior abnormalities; the PSSX for anterior, septal, inferior, and lateral walls. Lastly, the apical four chamber views are best suited for anterolateral, apical cap, septum and the two chambers for the anterior and inferior wall abnormalities.
View
Landmark
Area(s) Assessed
Anatomic Depiction6
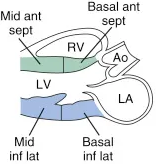
Parasternal Long Axis (PLAX)
4th-5th left intercostal space, probe marker to patient’s right shoulder
Anterior, inferior, and septal walls of the LV
Parasternal Short Axis (PSSX)
4th-5th left intercostal space, probe marker to patient’s left shoulder
Anterior, septal, inferior, and lateral walls of the LV
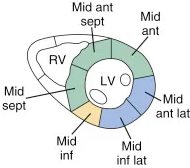
Apical Four Chamber (A4C)
Point of maximal impulse, probe marker at ~3 o’clock
Anterior, lateral, septal walls, and apical cap. RV free wall can also be assessed
Apical Two Chamber (A2C)
Rotation of A4C 60º
Inferior and anterior LV
RWMA may be described as hypokinetic, dyskinetic or akinetic. Any findings should be corroborated in an orthogonal imaging plane. Please see videos 1-3 depicting hypokinetic, dyskinetic and akinetic segments, respectively.
Video 1. This parasternal short axis view shows a hypokinetic segment. Can you guess which one? The lateral wall is hypokinetic compared to the remaining walls.
Video 2. This apical 4 chamber shows a dyskinetic left ventricular wall. This patient had an underlying LBBB producing this, but if you look closely, the septal wall is hypokinetic, with the basal septal wall being akinetic.
Video 3. This parasternal short axis shows an akinetic segment of the left ventricle. Can you guess which one? The anterio-lateral walls here are akinetic, and quite thin in appearance, likely from a recently missed ACS.
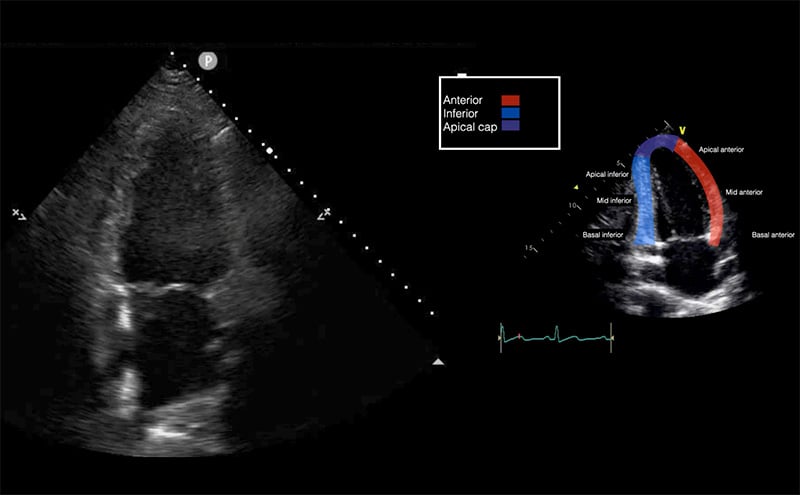
Corroborating TTE findings with EKG territorial changes may further help solidify the suspected diagnosis of AMI in patients. These findings are depicted in Figure 2.
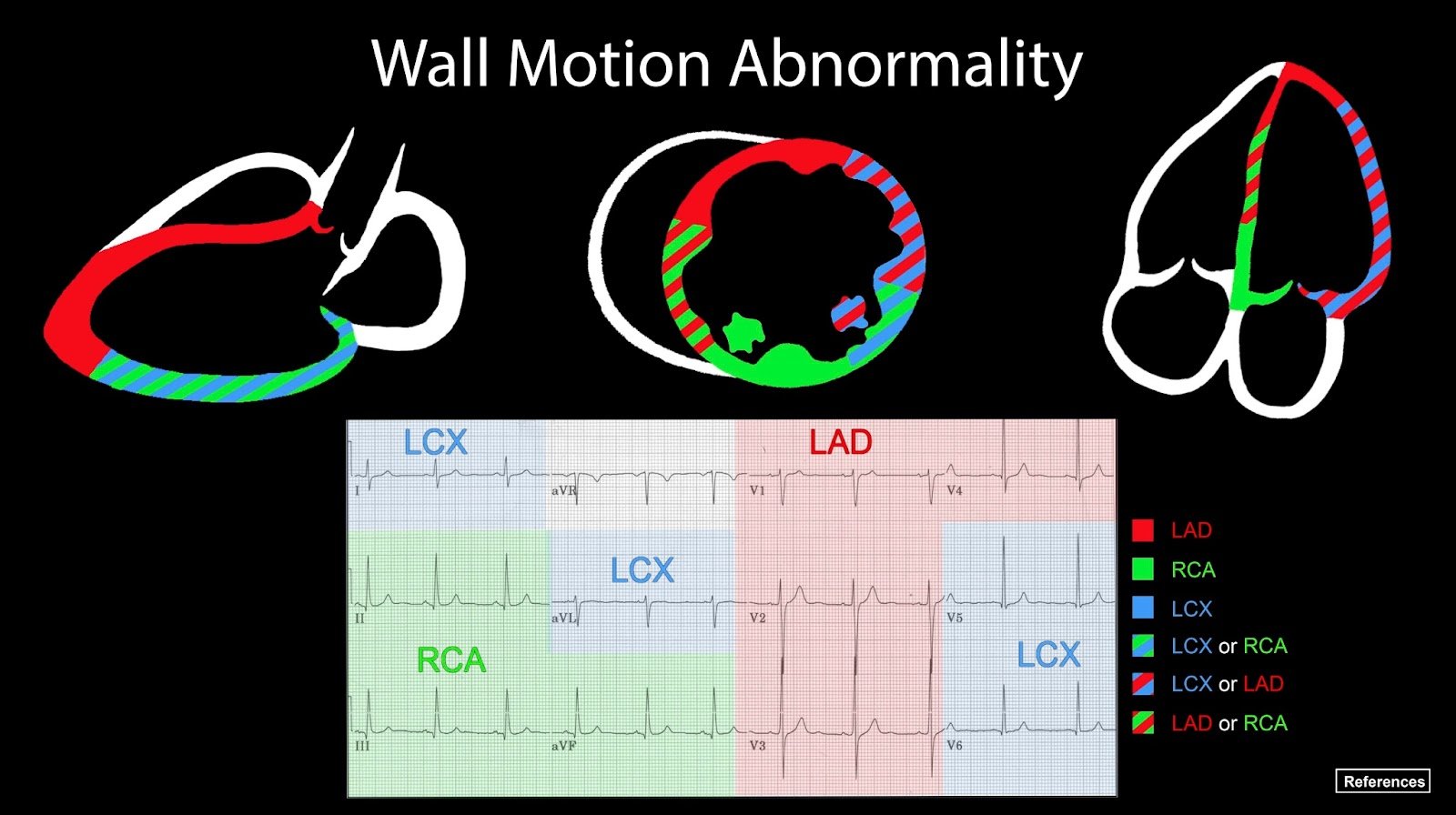
Figure 2. Vascular Territorial & EKG Distribution of the Left and Right Ventricle
It is important to consider chronic changes in patients with a history of known coronary artery disease. Acute vs. chronic findings can be delineated based on the thickness and echogenicity of the myocardium. Other causes of regional wall motion abnormalities include bundle branch or fascicular blocks, prior infarction, focal myocarditis, prior surgery, prosthetic valves, dysrhythmias (ventricular preexcitation) and stress cardiomyopathy.
Case Resolution
The patient was started on inotropic support with dobutamine to augment cardiac output after careful consideration that vasoactive agents may increase myocardial demand. Further investigations revealed an EKG demonstrating an anterolateral STEMI and an HS-TnT greater than 10,000 ng/L. She was emergently transferred to the cardiac center for immediate catheterization, which showed 100% occlusion of her proximal LAD. Post PCI, she was transferred to the cardiac care unit and extubated the following day. She was discharged from hospital.
References
- Kumar A, Connelly K, Vora K, et al. The Canadian Cardiovascular Society Classification of Acute Atherothrombotic Myocardial Infarction Based on Stages of Tissue Injury Severity: An Expert Consensus Statement. Can J Cardiol. 2024:40(1):1-14.
- Hauser AM, Gangadharan V, Ramos RG, et al. Sequence of mechanical, electrocardiographic and clinical effects of repeated coronary artery occlusion in human beings: echocardiographic observations during coronary angioplasty. J Am Coll Cardiol. 1985; 5:193.
- Wohlgelernter D, Cleman M, Highman HA, et al. Regional myocardial dysfunction during coronary angioplasty: evaluation by two-dimensional echocardiography and 12 lead electrocardiography. J Am Coll Cardiol. 1986; 7:1245.
- Sabia P, Afrookteh A, Touchstone DA, et al. Value of regional wall motion abnormality in the emergency room diagnosis of acute myocardial infarction. A prospective study using two-dimensional echocardiography. Circulation. 1991;84:I85.
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440.
- Diaz A, Ducharme A, Tardif JC: Echocardiography in acute coronary syndromes. In Théroux P, editor: Acute coronary syndromes , Saunders, Philadelphia, 2011.

